This is an incomplete question, here is a complete question.
A solution containing CaCl₂ is mixed with a solution of Li₂C₂O₄ to form a solution that is 3.5 × 10⁻⁴ M in calcium ion and 2.33 × 10⁻⁴ M in oxalate ion. What will happen once these solutions are mixed? Ksp (CaC₂O₄) = 2.3 × 10⁻⁹
A. Nothing will happen since both calcium chloride and lithium oxalate are soluble compounds.
B. Nothing will happen Ksp > Q for all possible precipitants.
C. A precipitate will form as calcium oxalate is not soluble to any extent.
D. A precipitate will form since Q > Ksp for calcium oxalate.
Answer : The correct option is, (D) A precipitate will form since Q > Ksp for calcium oxalate.
Explanation :
Concentration of
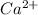 =
=
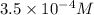
Concentration of
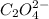 =
=
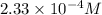
The equilibrium chemical reaction will be:
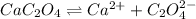
The solubility constant expression for this reaction is:
![K_(sp)=[Ca^(2+)][C_2O_4^(2-)]=2.3* 10^(-9)](https://img.qammunity.org/2021/formulas/chemistry/college/7nga199w3kt5zgw57ghg26kui95s6hrie0.png)
Now we have to calculate the ionic product for calcium oxalate.
![Q_(sp)=[Ca^(2+)][C_2O_4^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/y97q2yqvthal1v7sn80iup1oc4guvcvvvh.png)
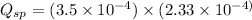
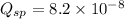
From this we conclude that,
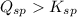 that means a white solid precipitate of calcium oxalate will be formed when the solutions are mixed.
that means a white solid precipitate of calcium oxalate will be formed when the solutions are mixed.
Hence, the correct option is, (D)