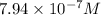Answer: The hydrogen ion concentration of his saliva is
Step-by-step explanation:
pH is defined as the negative logarithm of hydrogen ion concentration present in the solution.
To calculate the pH of the solution, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
We are given:
pH of the saliva = 6.1
Putting values in above equation, we get:
![6.1=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/zzh8tirun6x7dkwhj2z08iby3j3sluwzgf.png)
![[H^+]=10^(-6.1)=7.94* 10^(-7)M](https://img.qammunity.org/2021/formulas/chemistry/college/qjt6bhvdiuo1hym8nal5gvx6fwn6j0j7nu.png)
Hence, the hydrogen ion concentration of his saliva is