Answer:
The maximum kinetic energy of photoelectrons is 3.02 eV
Step-by-step explanation:
Given:
Wavelength of light
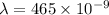 m
m
Work function
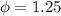 eV
eV
From the theory of photoelectric effect,
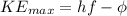
Where
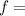 frequency of light,
frequency of light,
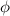 = work function its depends on nature of metal.
= work function its depends on nature of metal.
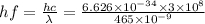
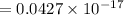
Now we convert energy in terms of electrovolt,
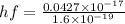
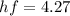 eV
eV
Put above value,
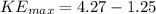
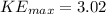 eV
eV
Therefore, the maximum kinetic energy of photoelectrons is 3.02 eV