Answer: The theoretical yield of the lithium chlorate is 1054.67 grams
Step-by-step explanation:
To calculate the mass for given number of moles, we use the equation:

Actual moles of lithium chlorate = 9.45 moles
Molar mass of lithium chlorate = 90.4 g/mol
Putting values in above equation, we get:
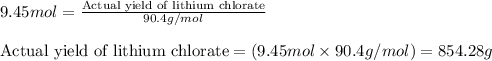
To calculate the theoretical yield of lithium chlorate, we use the equation:
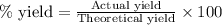
Actual yield of lithium chlorate = 854.28 g
Percentage yield of lithium chlorate = 81.0 %
Putting values in above equation, we get:

Hence, the theoretical yield of the lithium chlorate is 1054.67 grams