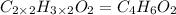Answer:
The molecular formula of the compound :
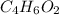
Step-by-step explanation:
The empirical formula of the compound =
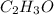
The molecular formula of the compound =
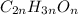
The equation used to calculate the valency is :
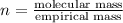
We are given:
Mass of molecular formula = 86 g/mol
Mass of empirical formula = 43 g/mol
Putting values in above equation, we get:
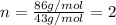
The molecular formula of the compound :