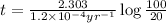The question is incomplete, here is the complete question:
The decay constant for 14-C is
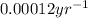 In 1947, the famous cave paintings in Lascaux, France were discovered and testing revealed that charcoal in the cave contained 20% of the 14-C found in living trees. Write a formula for the age of the charcoal (hence of the associated paintings). Show your work to find this formula.
In 1947, the famous cave paintings in Lascaux, France were discovered and testing revealed that charcoal in the cave contained 20% of the 14-C found in living trees. Write a formula for the age of the charcoal (hence of the associated paintings). Show your work to find this formula.
Answer: The formula for the age of the charcoal is
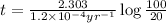
Step-by-step explanation:
Carbon-14 isotope is a radioisotope and its decay process follows first order kinetics.
Rate law expression for first order kinetics is given by the equation:
![k=(2.303)/(t)\log([A_o])/([A])](https://img.qammunity.org/2021/formulas/chemistry/college/bbi6c2ny1tf8wlzntta3i570f6pal714ld.png)
where,
k = rate constant =
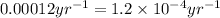
t = time taken for decay process = ? yr
![[A_o]](https://img.qammunity.org/2021/formulas/physics/college/3jrctnxyrdjmiz9ngr0s6o9r3hdvpo6qhe.png) = initial amount of the sample = 100 grams
= initial amount of the sample = 100 grams
[A] = amount left after decay process = (100 - 20) = 80 grams
Putting values in above equation, we get:
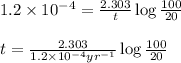
Hence, the formula for the age of the charcoal is