Answer:
Calculated molarity of acid is higher than the actual concentration.
Step-by-step explanation:
For an acid-base titration, the following equation is obeyed for calculation-
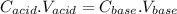
where, C represents concentration and V represents volume.
So,
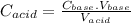
Here,
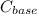 and
and
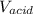 are constants.
are constants.
Actual volume of base added from burette is lesser than the volume used (based on assumption) for calculation of concentration of acid.
So, actual value of
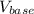 is lesser. Hence calculated concentration or molarity of acid is higher than the actual concentration.
is lesser. Hence calculated concentration or molarity of acid is higher than the actual concentration.