Answer:
- Element oxidized: N.
- The element reduced: Cr.
- The oxidizing agent: Chromium.
- The reducing agent: Nitrogen.
- Formula of the oxidizing agent: (CrO₄)²⁻
- Formula of the reducing agent: NO.
- name of the element oxidized: nitrogen.
- name of the element reduced: chromium.
Step-by-step explanation:
Hello,
In this case, the redox reaction is:
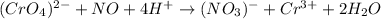
In such a way, each element has the following oxidation state distribution:
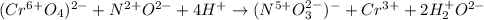
Thus, it seen that:
- The oxidized element is nitrogen as its oxidation state changes from 2+ to 5+. In addition, it is the reducing agent since it undergoes oxidation.
- The reduced element is chromium as its oxidation state changes from 6+ to 3+. In addition, it is the oxidizing agent since it undergoes reduction.
Hence, in order to answer:
- Element oxidized: N.
- The element reduced: Cr.
- The oxidizing agent: Chromium.
- The reducing agent: Nitrogen.
- Formula of the oxidizing agent: (CrO₄)²⁻
- Formula of the reducing agent: NO.
- name of the element oxidized: nitrogen.
- name of the element reduced: chromium.
Best regards.