Answer : The volume of the gas remaining is 56.5 liters.
The gas is hydrochloric acid and the formula of the gas is HCl.
The mass of
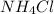 produced is, 110.7 grams.
produced is, 110.7 grams.
Explanation :
The balanced chemical reaction will be:
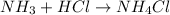
First we have to calculate the moles of
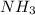 and HCl
and HCl
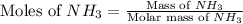
Molar mass of NH_3 = 17 g/mole
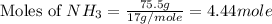
and,
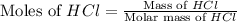
Molar mass of HCl = 36.5 g/mole
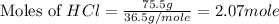
Now we have to calculate the limiting and excess reagent.
From the balanced reaction we conclude that
As, 1 mole of HCl react with 1 mole of
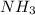
So, 2.07 mole of HCl react with 2.07 mole of
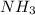
From this we conclude that,
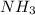 is an excess reagent because the given moles are greater than the required moles and HCl is a limiting reagent and it limits the formation of product.
is an excess reagent because the given moles are greater than the required moles and HCl is a limiting reagent and it limits the formation of product.
The remaining moles of HCl gas = 4.44 - 2.07 = 2.37 moles
Now we have to calculate the volume of the gas remaining.
Using ideal gas equation :
PV = nRT
where,
P = Pressure of gas = 752 mmHg = 0.989 atm (1 atm = 760 mmHg)
V = Volume of gas = ?
n = number of moles of gas = 2.37 moles
R = Gas constant = 0.0821 L.atm/mol.K
T = Temperature of gas =
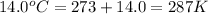
Putting values in above equation, we get:
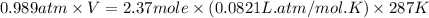
V = 56.5 L
Now we have to calculate the moles of
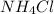
As, 1 mole of HCl react with 1 mole of
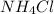
So, 2.07 mole of HCl react with 2.07 mole of
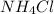
Now we have to calculate the mass of
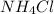
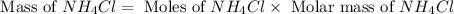
Molar mass of
 = 53.5 g/mole
= 53.5 g/mole
Thus, the volume of the gas remaining is 56.5 liters.
The gas is hydrochloric acid and the formula of the gas is HCl.
The mass of
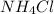 produced is, 110.7 grams.
produced is, 110.7 grams.