Answer:
COCl species is an intermediate in the mechanism.
Step-by-step explanation:
Intermediates are those chemical species which only appears for a short time in a course of a chemical reaction. They act as product and reactant during the reaction. Also they do not appear in overall chemical reaction.
Step 1:
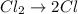
Step 2:
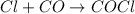
Step 3:
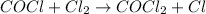
Overall reaction :
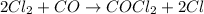
As we can see that all the chemicals are present in the overall reaction beside COCl.
COCl species is an intermediate in the mechanism.