Answer:
The mass ratio for chlorine is = 2 : 1
Step-by-step explanation:
Given that Compound A contains 8.43 g of chlorine for every 13.67 g of oxygen.
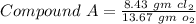
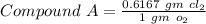 -------- (1)
-------- (1)
Similarly
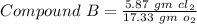 --------- (2)
--------- (2)
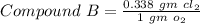
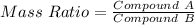 ------ (3)
------ (3)
Put the values from equation (1) & (2) in equation (3) we get
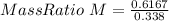
Mass Ratio = 2 : 1
Therefore the mass ratio for chlorine is = 2 : 1