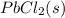Answer: The product formed in the solution is
Step-by-step explanation:
Double displacement reaction is defined as the reaction in which exchange of ions takes place.
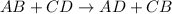
The chemical equation for the reaction of magnesium chloride and lead nitrate follows:
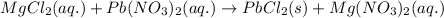
By Stoichiometry of the reaction:
1 mole of aqueous magnesium chloride reacts with 1 mole of aqueous lead nitrate to produce 1 mole of solid lead chloride and 1 mole of aqueous magnesium nitrate
Hence, the product formed in the solution is