a)
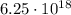 protons
protons
b)
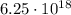 electrons
electrons
c) 1 electron
Step-by-step explanation:
a)
In this problem, the electric charge that we have is:
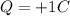
First of all, we observe that this charge is positive: this means that it will consist of protons.
In fact, protons are positively charged particles that reside in the nuclei of the atoms. The charge of one proton is
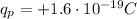
which is also known as fundamental charge.
Therefore, we can write the charge Q as consisting of the charge of several protons:
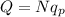
where N is the number of protons.
And solving for N,
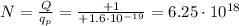
b)
Here the total charge is
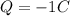
The total charge here is negative: this means that it consists of electrons. Electrons are negatively charged particles that orbit around the nucleus in an atom; the charge of one electron is
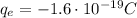
So, its charge is opposite to that of the proton.
Therefore, we can write the charge Q as the sum of the charges of N electrons:
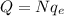
Where N is the number of electrons.
And solving for N, we find:
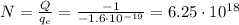
c)
In this case, the total net charge is
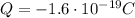
As in part b), we notice that the total charge is negative. Therefore, it will consist of N electrons (negatively charged particles), such that we have
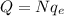
where
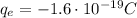 is the charge of one electron
is the charge of one electron
N is the number of electrons
And solving for N, we find:
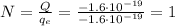
So, 1 electron.