Answer: In
 molecule, there are 2 atoms of hydrogen present.
molecule, there are 2 atoms of hydrogen present.
Step-by-step explanation:
Coefficients are defined as the numbers that are written in the front of atoms or molecules during a chemical reaction. They represent the number of moles of the substance present in a chemical reaction.
For Example:
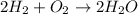
2 moles of hydrogen gas is reacting with 1 mole of oxygen gas to produce 2 moles of water molecule.
Subscript numbers are defined as the number of atoms of each element present in a compound.
For Example: In
 , 2 atoms of oxygen element is there and 1 atom of carbon element is there.
, 2 atoms of oxygen element is there and 1 atom of carbon element is there.
Superscript numbers are defined as the charges of ions reacting in a chemical reaction.
For Example: In
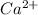 , 2+ charge is present on calcium ion.
, 2+ charge is present on calcium ion.
We are given:
A molecule of hydrogen which is

The number '2' is present in subscript, which represents the number of atoms in a molecule or a compound.
Hence, in
 molecule, there are 2 atoms of hydrogen present.
molecule, there are 2 atoms of hydrogen present.