Answer:
0.08moles
Step-by-step explanation:
Given parameters:
Volume of gas = 0.5L
Pressure of gas = 400kPa
Temperature of gas = 300K
Unknown:
Number of moles of N₂ = ?
Solution:
Applying the ideal gas law which is a combination of the three gas law: Boyle's law, Charles's law and Avogadro's law will solve this problem.
The ideal gas law is stated as;
PV = nRT
P is the pressure of the gas
V is the volume of the gas
n is the number of moles
R is the gas constant
T is the temperature of the gas
We need to convert kPa of the pressure to atm which is a more comfortable unit to work with.
400kPa will be
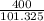 = 3.95atm
= 3.95atm
Input the variables in the equation;
3.95 x 0.5 = n x 0.082 x 300
n = 0.08moles