1.326 grams of NH3 are required to produce 4.65 g of HF.
Explanation:
Balanced chemical reaction is written first to know the number of moles taking part in original reaction.
NH3 +3 F2 ⇒3 HF + NF3
Given:
mass of HF = 4.65
First the number of moles of HF in 4.65 grams is calculated by using the formula:
number of moles (n) =
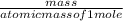
atomic mass of HF = 20 grams/mole
putting the values in the above equation number of moles can be found.
n =
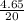
= 0.235 moles of HF are given.
From the equation it can be said that:
1 mole of NH3 reacts to form 3 moles of HF
so, x moles of NH3 would react to form 0.235 moles of HF
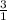 =
=
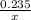
3x = 0.235
x =
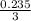
x = 0.078 moles of NH3 is required.
The moles are converted to mass by applying the formula:
mass = atomic mass X number of moles (atomic mass of NH3 = 17 grams/mole)
putting the values in the formula
mass = 17 X 0.078
mass = 1.326 grams