486 kPa will be the pressure of gas at 27 degrees.
Step-by-step explanation:
Data given :
Initial pressure of the gas P1 = 810 kPa
initial temperature of the gas T1 = 227 degrees OR 500.15 K
final pressure of the gas =?
final temperature of the gas = 27 degrees OR 300.15 K
Gay Lussac's law is used to calculate the pressure of the gas at 27 degrees or 300.15 K
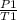 =
=
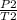
P2 =
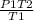
Putting the values in the equation:
P2 =
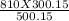
P2 = 486 KPa
486 Kpa is the pressure of the gas when temperature was reduced to 27 degrees.