10.4 is the pH of a solution made by dissolving 10 grams of sodium hydroxide in 1000 mL of Water.
Step-by-step explanation:
Data given:
volume of water or solution = 1000 ml or 1 L
mass of NaOH = 10 grams
molarity =?
pH =?
atomic mass of NaOH = 40 grams/mole
MOLARITY is calculated by using the formula:
molarity =
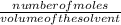
number of moles
n =
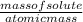
n =
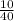
= 0.25 moles of NaOH
now molarity or concentration is calculated by using the formula:
molarity =
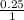
0.00025 M is the molarity.
pOH = - log(0.00025)
pOH = 3.60
pH is calculated as:
14 = pH + pOH
pH = 14-pOH
= 14 - 3.60
= 10.4 is the pH of the NaOH solution.