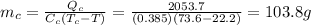Answer:
103.8 g
Step-by-step explanation:
When the hot piece of copper is placed in the water at lower temperature, the piece of copper gives off thermal energy to the water; as a result, the temperature of the copper decreases while the temperature of the water increases, until they both reach the equilibrium temperature.
The heat given off by the piece of copper is equal to the heat absorbed by the water, so we can write:
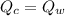
where:
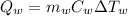 is the heat absorbed by the water, where
is the heat absorbed by the water, where
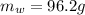 is the mass of water
is the mass of water
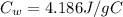 is the specific heat of water
is the specific heat of water
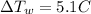 is the rise in temperature of the water
is the rise in temperature of the water
Solving,
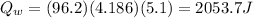
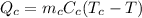 is the heat released by the copper, where
is the heat released by the copper, where
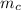 is the mass of copper
is the mass of copper
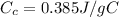 is the specific heat of copper
is the specific heat of copper
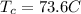 is the initial temperature of copper
is the initial temperature of copper
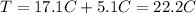 is the equilibrium temperature
is the equilibrium temperature
Solving for the mass,