Answer:
5.56g
Step-by-step explanation:
Given parameters:
Volume of H₂ = 5.55L
Pressure of H₂ = 288mmHg
Temperature of H₂ = 29.5°C
Unknown:
Mass of zinc = ?
Solution:
To start with, let us write the reaction equation of this simple displacement reaction:
Zn + 2HCl → ZnCl₂ + H₂
Now we have to work from the known or given compound to the unknown.
The known here is the hydrogen gas produced. From the parameters given, let us solve for the number of moles using the ideal gas law;
PV = nRT
take the given species to appropriate units;
288mmHg to atm gives;
760mmHg gives 1atm
288mmHg will give
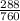 = 0.38atm
= 0.38atm
take the temperature to k;
29.5°C ; 29.5 + 273 = 302.5K
From the ideal gas equation:
P is the pressure
V is the volume
n is the number of moles
R is the gas constant
T is the temperature
Input the parameters and solve for n;
0.38 x 5.55 = n x 0.082 x 302.5
n = 0.085moles
From the balanced reaction equation;
1 mole of H₂ is produced from 1 mole of Zn
0.085moles of H₂ will be produced from 0.085 moles of Zn
Mass of Zn = number of moles of Zn x molar mass of Zn
= 0.085 x 65.4
= 5.56g