The specific heat of a material is 0.137 J/g°C.
Step-by-step explanation:
The specific heat formula relates the heat energy required to perform a certain reaction with the mass of the reactants, specific heat and the change in temperature during the reaction.
Q = mcΔT
Here m is the mass, Q is the heat energy required, ΔT is the change in temperature and c is the specific heat.
So, if we have to determine the specific heat of the object, then we have to determine the ratio of heat required to mass of the object with change in time, as shown below.
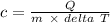
As mass of the object m is given as 35 g and the energy is said to be absorbed so Q = 96 J.
The temperature values given should be changed from kelvin to celsius first. So, initial temperature 293 K will become 293-273.15 = 19.85°C.
Similarly, the final temperature will be 313 - 273.15 = 39.85°C.
Then, ΔT = 39.85-19.85 = 20 °C
Then,
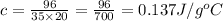
So, the specific heat of a material is 0.137 J/g°C.