Answer:
9.09 x 10²³ atoms
Step-by-step explanation:
Given parameters:
Volume of nitrogen = 33.9L
Condition of reaction = STP
Unknown:
Number of atoms of Copper reacting = ?
Solution:
Let us write the reaction equation first;
3Cu + N₂ → Cu₃N₂
We have to work from the know to unknown specie.
With the information given, let us find the number of moles of nitrogen since it is the known specie;
At STP;
Number of moles =
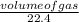 =
=
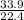 = 1.51 moles
= 1.51 moles
From the balanced equation;
1 mole of Nitrogen gas will give 1 mole of copper(II)nitride
1.51 moles of Nitrogen gas will produce 1.51 moles of copper(II)nitride
To find the number of atoms;
1 mole of a substance contains 6.02 x 10²³ atoms
1.51 moles of a substance will contain 1.51 x 6.02 x 10²³ atoms
= 9.09 x 10²³ atoms