Answer:
2M
Step-by-step explanation:
Given parameters:
Number of moles of FeCl₃ = 0.3 moles
Volume of water = 150mL
Unknown:
Molarity of solution = ?
Solution:
The molarity of a solution is the number of moles of solutes in a given solution. It is one of the ways of expressing concentration. The unit is always M i.e mol/dm³.
Molarity =
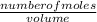
Express the volume in dm³;
150mL gives 0.15dm³
Molarity =
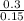
= 2M