0.103 litre is the volume of 0.100 M Pb(NO3)2 is required to react completely with 45.6 ml of 0.225 M HCI.
Step-by-step explanation:
Balance equation for the reaction:
Pb(NO3)2 + 2 HCI => PbCl2 + 2 HNO3
Data given:
Volume of Pb(NO3)2 V1 = ?
Molarity of Pb(NO3)2 M1= 0.1 M
Volume of HCl V2 = 45.6 ml
molarity of HCl M2 = 0.225 M
Using the formula for titration reaction between Pb(NO3)2 + 2 HCI
M1V1 = M2V2
V1 =
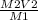
Putting values in the equation:
V1 =
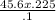
= 102. 6 ml
The nearest result from the options is 103 ml, so the volume of Pb(NO3)2 used is 103 ml.
Volume in litres will be converted by dividing 103 ml by 1000
The result is 0.103 litre is the volume of lead (II) nitrate.