Answer:
Step-by-step explanation:
Regardless of the value of the y subscript, the number of moles of CO₂ that can be produced from 1 mol of
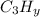 is 3 moles: that is so because every molecule of
is 3 moles: that is so because every molecule of
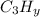 contains 3 atoms of C and every atom of C produces one molecule of CO₂.
contains 3 atoms of C and every atom of C produces one molecule of CO₂.
Then, you can calculate the number of moles of
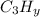 in 97.0g using the molar mass of the compound:
in 97.0g using the molar mass of the compound:
1. Molar mass of
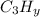 :
:
- 3 × 12g/mol + y × 1 g/mol = (36 + y)g/mol
2. Number of moles = mass in grams / molar mass
- Number of moles of
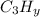 =
=
= 97.0 g / (36 + y)g/mol = 97.0/(36+y) mol
3. Number of moles of CO₂
As stated above, the number of moles of CO₂ is 3 times the number of moles of
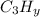 :
:
- Number of moles of CO₂ = 3 × 97.0 / (36 + y) mol = 291 / (36 + y)
For instance, imagine the compound is C₃H₈. How many moles of CO₂ will be produced from 97.0 g of C₃H₈?
You can replace 8 for y:
- 291 / (36 + 8) mol = 291.0 / (44) mol = 6.61 mol