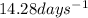The question is incomplete, here is the complete question:
Recall that m(t) = m.(1/2)^t/h for radioactive decay, where h is the half-life. Suppose that a 500 g sample of phosphorus-32 decays to 356 g over 7 days. Calculate the half life of the sample.
Answer: The half life of the sample of phosphorus-32 is
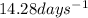
Explanation:
The equation used to calculate the half life of the sample is given as:
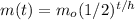
where,
m(t) = amount of sample after time 't' = 356 g
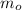 = initial amount of the sample = 500 g
= initial amount of the sample = 500 g
t = time period = 7 days
h = half life of the sample = ?
Putting values in above equation, we get:
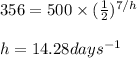
Hence, the half life of the sample of phosphorus-32 is