Answer:
182mL
Step-by-step explanation:
Given parameters:
Molarity of solution = 3.11M
New volume = 232mL = 0.232dm³
New molarity = 2.44M
Unknown:
Volume of original solution = ?
Solution:
We know the full information about the new solution we want to obtain. From this, we should find the number of moles.
Using the number of moles, we can find the original volume.
Molarity =
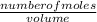
New number of moles = molarity x volume = 2.44 x 0.232
= 0.57moles
The original volume will be;
Volume =
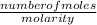 =
=
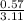 = 0.182dm³ or 182mL
= 0.182dm³ or 182mL