5.426 x
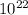 molecules are there in a deep breath of air whose volume is 2. 35 L at body temperature 36 C and a pressure of 740 torr.
molecules are there in a deep breath of air whose volume is 2. 35 L at body temperature 36 C and a pressure of 740 torr.
Step-by-step explanation:
Data given:
volume V = 2.35 L
Temperature T = 36 Degrees or 273.15 + 36 = 309.15 K
pressure P = 740 torr OR 0.973 Atm
R = 0.08205 L atm / mole K
n (number of molecules of gas)= ?
The formula used will be
PV = nRT
Putting the value of given variables in above equation:
n =
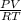
=
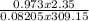
= 0.0901 moles
According to Avagadro' s law that gases at equal volume, temperature and pressure contains same molecules.
number of molecules:
number of moles x avagadro number
0.0901 x 6.02 x

= 0.54 x

= 5.426 x
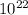 molecules.
molecules.