14 moles of NH3 can be produced from 21.0 mol of H2 and excess N2.
Step-by-step explanation:
Balanced equation for the production of ammonia is given as:
3H2(g)+N2(g)→2NH3(g)
Given that:
number of moles of H2 = 21 moles
moles of NH3 produced from 21 moles of H2 =?
In the above equation the limiting reagent is hydrogen gas so the production of ammonia directly depends on it.
From the chemical equation we can see that:
3 moles of H2 react to form 2 moles of NH3
so, 21 moles of H2 will react to form x moles of NH3
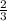 =
=
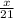
=
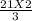
= 14 MOLES
from the calculation by applying stoichiometry it is found that 14 moles of ammonia is produced by using 21 moles of hydrogen.