0.208 is the specific heat capacity of the metal.
Step-by-step explanation:
Given:
mass (m) = 63.5 grams 0R 0.0635 kg
Heat absorbed (q) = 355 Joules
Δ T (change in temperature) = 4.56 degrees or 273.15+4.56 = 268.59 K
cp (specific heat capacity) = ?
the formula used for heat absorbed and to calculate specific heat capacity of a substance will be calculated by using the equation:
q = mc Δ T
c =
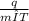
c =
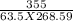
= 0.208 J/gm K
specific heat capacity of 0.208 J/gm K
The specific heat capacity is defined as the heat required to raise the temperature of a substance which is 1 gram. The temperature is in Kelvin and energy required is in joules.