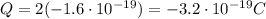Answer:
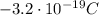
Explanation:
- Protons are positively charged particles contained in the nucleus of the atom. They have a charge of
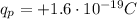
- Electrons are negatively charged particles orbiting around the nucleus of the atom. They have a charge of
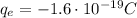
For a neutral atom, the number of protons is equal to the number of electrons, so the total charge of a neutral atom is:
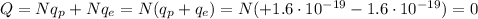
In this problem, we have an atom having an excess of 2 electrons, i.e. it has 2 more electrons than protons.
Therefore, the charge of this atom will be:
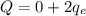
and substittuing, we find