Answer:
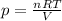
Step-by-step explanation:
The ideal gas law equation is an equation that relates some of the quantities that describe a gas: pressure, volume and temperature.
The equation is:
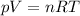
where
p is the pressure of the gas
V is the volume of the gas
n is the number of moles of the gas
R is the gas constant
T is the absolute temperature of the gas (must be expressed in Kelvin)
Here we want to solve the equation isolating p, the pressure of the gas.
We can do that simply by dividing both terms by the volume, V. We find:
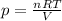
So, we see that:
- The pressure is directly proportional to the temperature of the gas
- The pressure is inversely proportional to the volume of the gas