Answer: The pH of acid solution is 4.58
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
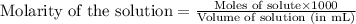 .....(1)
.....(1)
Molarity of KOH solution = 1.1000 M
Volume of solution = 41.04 mL
Putting values in equation 1, we get:

Molarity of propanoic acid solution = 0.6100 M
Volume of solution = 224.9 mL
Putting values in equation 1, we get:
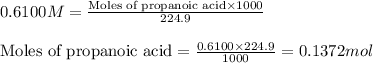
The chemical reaction for propanoic acid and KOH follows the equation:
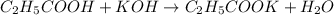
Initial: 0.1372 0.04514
Final: 0.09206 - 0.04514
Total volume of solution = [224.9 + 41.04] mL = 265.94 mL = 0.26594 L (Conversion factor: 1 L = 1000 mL)
To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(\frac{[\text{salt}]}{[acid]})](https://img.qammunity.org/2021/formulas/chemistry/college/ig55txdkd4hbzffv44i621gg6vfsxwc8y3.png)
![pH=pK_a+\log(([C_2H_5COOK])/([C_2H_5COOH]))](https://img.qammunity.org/2021/formulas/chemistry/college/yxbiyhdsv7kwnchh8jwkvllfftuza8rkc0.png)
We are given:
 = negative logarithm of acid dissociation constant of propanoic acid = 4.89
= negative logarithm of acid dissociation constant of propanoic acid = 4.89
![[C_2H_5COOK]=(0.04514)/(0.26594)](https://img.qammunity.org/2021/formulas/chemistry/college/pdzhz0urysp90c58jh1tz4urx372tp3hrg.png)
![[C_2H_5COOH]=(0.09206)/(0.26594)](https://img.qammunity.org/2021/formulas/chemistry/college/uqko0s51j65za7uecadakagnaiu0lt17lw.png)
pH = ?
Putting values in above equation, we get:
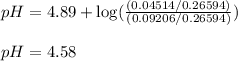
Hence, the pH of acid solution is 4.58