Answer:
The thorium-232 is 1.45 billion years old.
Step-by-step explanation:
Given:
Let original mass of thorium-232 be
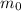 .
.
Half life for the decay process is,
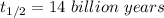
Mass of thorium-232 left after some time (m) =
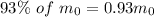
Let the rate of decay be 'r' and time taken be 't' billion years.
We know that, the amount of radioactive element left after 't' years is given by the formula:
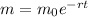
Also, the rate of decay is given by the formula:
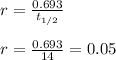
Now, plug in all the given values and solve for 't'. This gives,
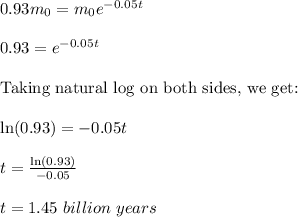
Therefore, the thorium-232 is 1.45 billion years old.