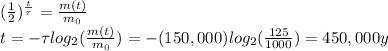Answer:
450,000 years
Step-by-step explanation:
The equation that describes the decay of a radioactive isotope is:
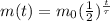
where
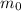 is the mass of the isotope at time t = 0
is the mass of the isotope at time t = 0
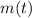 is the mass of the isotope at time t
is the mass of the isotope at time t
 is the half-life of the isotope, which is the time it takes for the isotope to halve its mass
is the half-life of the isotope, which is the time it takes for the isotope to halve its mass
In this problem:
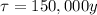 is the half-life of the radioisotope
is the half-life of the radioisotope
m(t) = 125 g is the mass of radioisotope left after time t
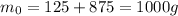 is the initial mass of the radioisotope (the sum of the mass of the final radioisotope + the mass of the daughter nuclei, since mass is conserved)
is the initial mass of the radioisotope (the sum of the mass of the final radioisotope + the mass of the daughter nuclei, since mass is conserved)
So, we can re-arrange the equation to find t: