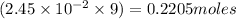Answer:
For A: The moles of oxygen atoms are 13.24 moles
For B: The moles of oxygen atoms are 86.8 moles
For C: The moles of oxygen atoms are 0.2205 moles
Step-by-step explanation:
- For A: 3.31 moles
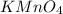
1 mole of
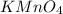 contains 1 mole of potassium atoms, 1 mole of manganese atoms and 4 moles of oxygen atoms
contains 1 mole of potassium atoms, 1 mole of manganese atoms and 4 moles of oxygen atoms
So, moles of oxygen atoms = (3.31 × 4) = 13.24 moles
- For B: 43.4 moles

1 mole of
 contains 1 mole of carbon atoms and 2 moles of oxygen atoms
contains 1 mole of carbon atoms and 2 moles of oxygen atoms
So, moles of oxygen atoms = (43.4 × 2) = 86.8 moles
- For C:
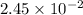 moles
moles
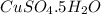
1 mole of
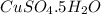 contains 1 mole of copper atoms, 1 mole of sulfur atoms, 10 moles of hydrogen atoms and 9 moles of oxygen atoms
contains 1 mole of copper atoms, 1 mole of sulfur atoms, 10 moles of hydrogen atoms and 9 moles of oxygen atoms
So, moles of oxygen atoms =