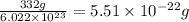Answer:
Number of silver ions :
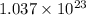
Number of chromate ions :
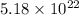
Mass of single silver chromate :
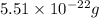
Step-by-step explanation:
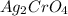 , Silver chromate
, Silver chromate
Mass of silver chromate = 28.6 g
Molar mass of silver chromate = 332 g/mol
Moles of silver chromate =
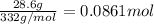
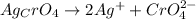
1 mole of silver chromate has 2 mole of silver ions and 1 mole of chromate ions.
Then silver ions in 0.0861 moles of silver chromate :
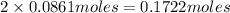 of silver ion
of silver ion
Then chromate ions in 0.0861 moles of silver chromate :
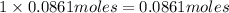 of chromate ion
of chromate ion
Number of silver ions :
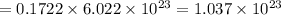
Number of chromate ions :
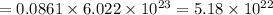
Mass of 1 mole of silver chromate = 332 g
1 mole=
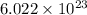 molecules/atoms/ions
molecules/atoms/ions
Mass of single silver chromate :