-20.16 KJ of heat are released by the reaction of 25.0 g of Na2O2.
Step-by-step explanation:
Given:
mass of Na2O2 = 25 grams
atomic mass of Na2O2 = 78 gram/mole
number of mole =

=
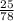
=0. 32 moles
The balanced equation for the reaction:
2 Na2O2(s) + 2 H2O(l) → 4 NaOH(aq) + O2(g) ∆Hο = −126 kJ
It can be seen that 126 KJ of energy is released when 2 moles of Na2O2 undergoes reaction.
similarly 0.3 moles of Na2O2 on reaction would give:
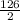 =
=
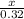
x =
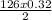
= -20.16 KJ
Thus, - 20.16 KJ of energy will be released.