Answer:
Q = 37855.77 J
Step-by-step explanation:
given data
mass = 15 gram = 0.015 kg
temperature t1 = 100°C
temperature t2 = 37°C
solution
as heat is absorbed by steam to liquid is
Q1 = mL ............1
and
heat absorbed by 100 degree C water to body temperature so
Q2 = m c ΔT .......2
so that here total head absorbed is
total hat absorbed is
Q = Q1 + Q2 ...........3
put her value
Q = mL + m c ΔT
Q = 0.015 × 2.26 ×
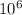 J/kg + 0.015 × 4186 × (100 - 37 )
J/kg + 0.015 × 4186 × (100 - 37 )
Q = 37855.77 J
Q = 9.045 cal