100.133 degree celsius is the boiling point of the solution formed when 15.2 grams of CaCl2 dissolves in 57.0 g of water.
Step-by-step explanation:
Balanced eaquation for the reaction
CaCl2 + 2H20 ⇒ Ca(OH)2 + HCl
given:
mass of CaCl2 = 15.2 grams
mass of the solution = 57 grams
Kb (molal elevation constant) = 0.512 c/m
i = vont hoff factor is 1 as 1 mole of the substance is given as product.
Molality is calculated as:
molality =
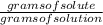
=
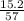
= 0.26 M
Boiling point is calculated as:
ΔT = i x Kb x M
= 1 x 0.512 x 0.26
= 0.133 degrees
The boiling point of the solution will be:
100 degrees + 0.133 degrees (100 degrees is the boiling point of water)
= 100.133 degree celcius is the boiling point of mixture formed.