a) Molarity of methanol is 1.24 M or 0.19 moles/0.153 litres.
b) molarity of CaCl2 is 0.37 M or 0.084 moles/ 0.2244litres.
c) molarity of napthalene is 0.81 M or 0.069 moles/0.085 litres.
Step-by-step explanation:
The formula used in the calculation of molarity is:
molarity =
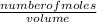 equation 1
equation 1
number of moles can be calculated as:
number of moles =
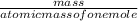 equation 2
equation 2
a) mass of methanol = 6.19 grams
volume of the solution = 1.50 x 102 ml= 153 ml or 0.153 L
atomic mass of methanol = 32.04 gram/mole
number of moles will be calculated by putting the given values in equation 2
number of moles =
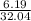
= 0.19 moles
molarity is known by putting the values in equation 1
molarity =
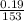
= 1.24 M
b) mass of CaCl2 = 9.43 gram
volume of the solution= 224 ml or 0.2244 litres
atomic mass of CaCl2 = 110.8 gram/mole
number of moles is calculated using equation 2
number of moles =
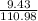
= 0.084 moles
molarity is calculated using equation 1
molarity =
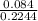
= 0.37 M
c) mass = 8.86 gram
volume = 85.2 ml or 0.08512 L
atomic mass = 128.17
number of moles is calculated by putting the values in equation2:
number of moles =
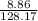
= 0.069 moles
molarity =
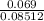
= 0.80 M