Water decomposes when electrolyzed to produce hydrogen and oxygen gas. If 2.5 grams of water were decomposed 1.04 grams of oxygen will be formed.
BCA table:
2
 O ⇒
O ⇒
 +
+

B 0.13 0 + 0
C -0.13 0.065 + 0.065
A 0 0.065
Step-by-step explanation:
Balanced equation for water decomposition into hydrogen and oxygen gases
2
 O ⇒
O ⇒
 +
+

B 0.13 0 + 0
C -0.13 0.065 + 0.065
A 0 0.065
Number of moles of water =

mass = 2.5 grams
atomic mass= 18 grams
number of moles can be known by putting the values in the formula,
n =
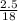
= 0.13 moles
2 moles of water gives one mole of oxygen on decomposition
so, 0.13 moles of water will give x moles of oxygen on decompsition
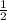 =
=
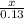
x = 0.065 moles of oxygen will be formed.
moles to gram will be calculated as
mass =number of moles x atomic mass
= 0.065 x 16
= 1.04 grams of oxygen.