382.75 is the temperature of the 0.750 mol of an ideal gas occupy a volume of 35.9 L at 114 kPa.
Step-by-step explanation:
Given:
number of moles (n) = 0.750
volume of the gas = 35.9 litres
pressure on the ideal gas = 114 kPa or 1.125 atm
R (gas constant) = 0.0821J/Kmole
Absolute temperature of gas in celcius =?
the equation for the ideal gas is
PV = nRT
putting the values in the above equation:
T =
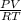
=
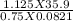
= 655.907
655.9 kelvin is the temperature of the ideal gas.
To convert this unit of kelvin into degree celcius:
K-273.15 = C
655.9 - 273.15
= 382.75 degrees
382.75 degrees celcius is the temperature of the ideal gas.