Answer:
6.47 grams is the maximum mass of carbon dioxide that could be produced by the chemical reaction.
Step-by-step explanation:
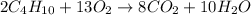
Moles of butane =
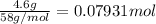
Moles of oxygen gas =
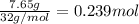
According to reaction , 13 moles of oxygen gas reacts with 2 moles of butane,then 0.239 moles of oxygen gas will react with :
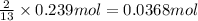 of butane.
of butane.
From this we can see that butane is present in an excess amount and oxygen gas is present in limited amount. Hence, oxygen gas is a limiting reagent.
Since, oxygen gas is a limiting reagent which means that amount of carbon dioxide gas produce will depend upon moles of oxygen gas.
According to reaction, 13 moles of oxygen gas gives 8 moles of carbon dioxide, then 0.239 moles of oxygen gas will give:
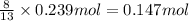
Mass of 0.1471 moles of carbon dioxide gas:
0.147 mol × 44.01 g/mol = 6.47 g
6.47 grams is the maximum mass of carbon dioxide that could be produced by the chemical reaction.