Answer:
From the highest to the lowest
Third excited state (n=4)
 Second excited state (n=3)
Second excited state (n=3)
 First excited state (n=2)
First excited state (n=2)
 Ground state (n=1)
Ground state (n=1)
Step-by-step explanation:
The Bohr Model, by Niels Bohr, is a planetary model in which the negatively charged electrons orbit a small, positively charged nucleus similar to the planets orbiting the sun. Niels Bohr in his model states that:
- The electron is able to revolve in certain stable orbits around the nucleus without radiating any energy
- Electrons can only gain and lose energy by jumping from one allowed orbit to another, absorbing or emitting electromagnetic radiation with a frequency ν determined by the energy difference of the levels according to the Planck relation:
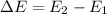 = hv, where h = planck's constant.
= hv, where h = planck's constant.
The lowest value of n is 1, which is the ground state
n = principal quantum number
Therefore, from the highest to the lowest, the energy levels are ranked as:
Third excited state (n=4)
Second excited state (n=3)
First excited state (n=2)
Ground state (n=1)