Answer:
If temperature stays the same, the amount of carbon dioxide dissolved in the ocean would increase.
Step-by-step explanation:
The carbon dioxide in the air (
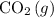 ) and the carbon dioxide dissolved in the ocean (
) and the carbon dioxide dissolved in the ocean (
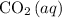 ) are in a solution equilibrium:
) are in a solution equilibrium:
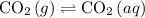 .
.
Assume that the concentration of carbon dioxide in the air (
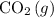 ) increased while temperature stayed the same.
) increased while temperature stayed the same.
By Le Ch
 atelier's Principle, the solution equilibrium
atelier's Principle, the solution equilibrium
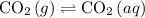 would shift to offset this increase in
would shift to offset this increase in
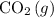 concentration. Specifically, this equilibrium would reduce the amont of
concentration. Specifically, this equilibrium would reduce the amont of
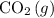 in the system by converting more atmospheric
in the system by converting more atmospheric
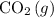 to
to
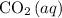 dissolved in the ocean. Therefore, the concentration of
dissolved in the ocean. Therefore, the concentration of
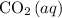 dissolved in the ocean would increase.
dissolved in the ocean would increase.