Answer:
0.228 L is the volume that the gas occupies after it is driedand stored at STP.
Step-by-step explanation:
Pressure of the wet gas =
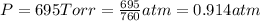
1 atm = 760 Torr
Pressure of water vapor = p = 0.0372 atm
Pressure of gas =

Volume of wet gas =
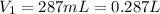
1 mL = 0.001 L
Temperature of the wet gas =
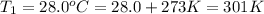
Pressure of dry gas at STP =
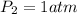
Temperature of the dry gas at STP ,
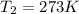
Volume of dry gas at STP =

Using combined gas law:
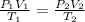
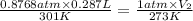
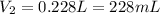
0.228 L is the volume that the gas occupies after it is driedand stored at STP.