Answer:
0.000241 M is the molar solubility of
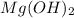 in pure water.
in pure water.
Step-by-step explanation:
Solubility product of metal hydroxide =
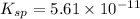
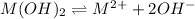
S 2S
The expression of a solubility product is given by :
![K_(sp)=[M^(2+)][OH^-]^2](https://img.qammunity.org/2021/formulas/chemistry/high-school/ctwqec203r8l6uvvbi02tcriq8t83re5r7.png)
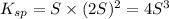
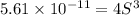
Solving for S:
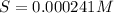
0.000241 M is the molar solubility of
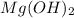 in pure water.
in pure water.