Answer: (1). If calcium bromide is dissolved in water you can say that the equilibrium concentrations of calcium and bromide ions are high.
(2). The solubility of calcium bromide in water is high.
Step-by-step explanation:
We know that for a reaction its solubility product is equal to the product of concentration of ions present.
For example,
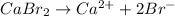
![K_(sp) = [Ca^(2+)][Br^(-)]^(2)](https://img.qammunity.org/2021/formulas/chemistry/college/lokh4naq7q4jfkb0cklxwqm72m99ytld6v.png)
As it is given that for this reaction
 > 1. Hence, the product of concentration of both these ions will be greater than 1 which means that products are favored.
> 1. Hence, the product of concentration of both these ions will be greater than 1 which means that products are favored.
Therefore, equilibrium concentrations of calcium and bromide ions are high.
Also, more is the number of these ions formed more will be the solubility of the compound (
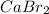 ).
).
As a result, the solubility of calcium bromide in water is high.