Answer:
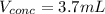
Step-by-step explanation:
Hello,
In this case, a problem is about dilution, which is a process wherein from a concentrated acid, a less concentrated solution is obtained via adding an extra volume. In such a way, since the required acid has a pH of 1.50, it means that it has a hydrogen ions concentration of:
![[H]^+=10^(-pH)=10^(-1.50)=0.0316M](https://img.qammunity.org/2021/formulas/chemistry/high-school/ps1f3u7sbwwusyc663zxcc2pvtzjzlaf4q.png)
Thus, since nitric acid is a strong acid, the concentration of hydrogen ions, equals the concentration is the acid due to complete dissociation, hence:
![[H]^+=[HNO_3]=0.0316M](https://img.qammunity.org/2021/formulas/chemistry/high-school/xqdqb5vbhf9znotr3m1uwc85263vezk3b1.png)
Thereby, it is concentration of the diluted acid. Now, as during a dilution process the moles of the acid are kept constant we obtain:
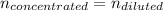
That in terms of molarities and volume result:
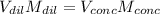
Thus, solving for the used volume of concentrated acid, we obtain:
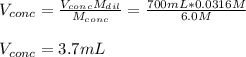
Best regards.